Transversal Translational Medicine
Activities
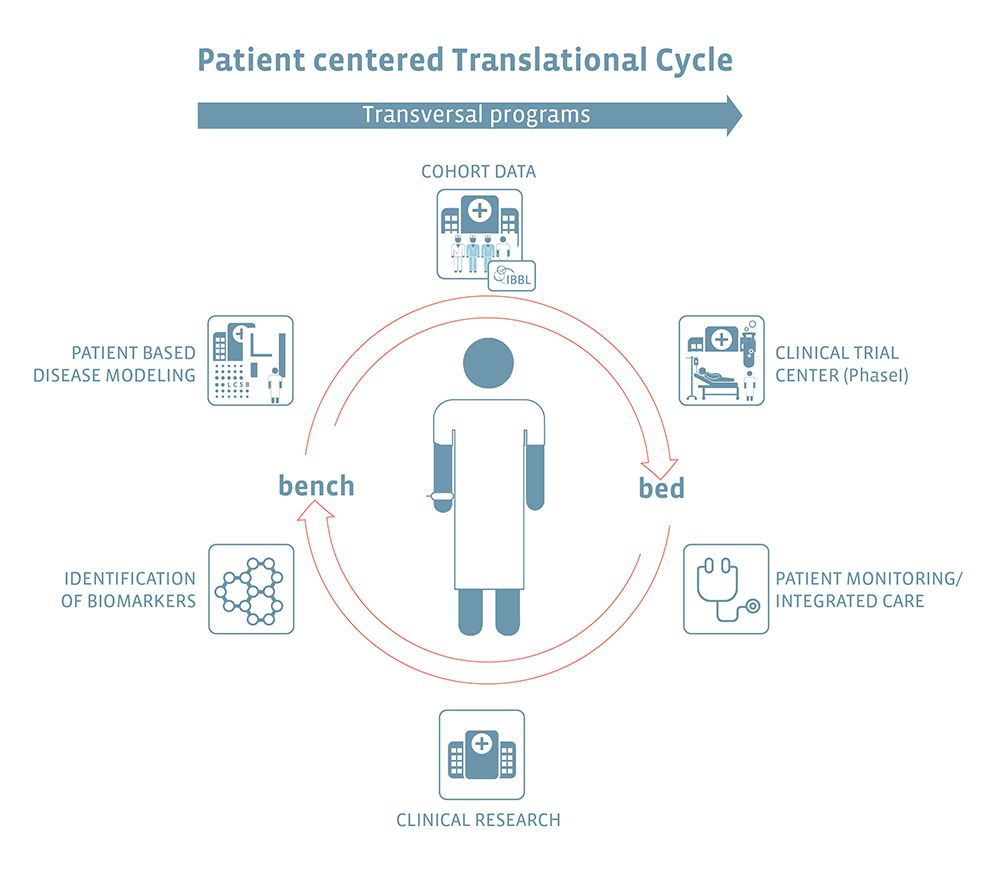
Transversal Translational Medicine (TTM) at the LIH enhances the ability of biomedical stakeholder institutions to work translationally. TTM aims to foster bed-to-bench-to-bed collaborations within LIH, inter-institutionally across Luxembourg and internationally, in view of implementing translational programmes across research topics, supported by specific platforms and infrastructures bridging between fundamental research and real-world healthcare. With the longstanding experience of the Luxembourgish National Centre for Excellence in Research (Parkinson’s disease) (NCER-PD) programme serving as a blueprint for translational bed-to-bench-to-bed cycles, TTM is perfectly positioned to enhance and develop translational initiatives in biomedical research and beyond.

Clinical research
Clinical research studies carried out on healthy and diseased individuals aim to improve knowledge of diseases, advance diagnostics and develop new treatments or medical devices to ensure better patient care.
Identification of biomarkers
Clinical research allows the identification of prognostic or diagnostic biomarkers, i.e. measurable indicators of the severity or presence of a disease.

Cohort data
Biological, genetic and clinical data are collected from cohort study participants, with the support of research infrastructures such as the Integrated Biobank of Luxembourg (IBBL), to investigate disease causes and establish links between risk factors and health outcomes.
Bioinformatics Core group, which enables other researchers from different backgrounds to efficiently manage, analyse and interpret their data.
ELIXIR-LU, the Luxembourgish node of ELIXIR, the European infrastructure for life science information, which focuses on the long-term sustainability of tools and data for Translational Medicine. Translational Medicine data integrates clinical information with molecular and cellular data for a better understanding of diseases
Clinical trial center (phase I)
New candidate drugs or medical interventions are tested and their dosage determined on small groups of patients during Phase I clinical trials.
Patient monitoring/integrated care
New therapies/interventions are administered to patients and their efficacy monitored, in collaboration with all healthcare stakeholders, resulting in integrated patient-centric care.
Contact