Clinical and Epidemiological Investigation center
Dr Manon GantenbeinTranslational Medicine Operations Hub
The operational units of the LIH (CPMO, CIEC, CCMS, IBBL) and its collaborative platforms (LCTR, DMSP, LuxGen, RPP) have been consolidated in the Translational Medicine Operations Hub (TMOH), in order to integrate and optimise their accessibility, workflows and project/portfolio oversight.
The new department ensures a full support to clinical research from the planning of the clinical research studies to their execution and closure via the collection, processing, storage and analysis of high-quality biological samples and structured clinical data.
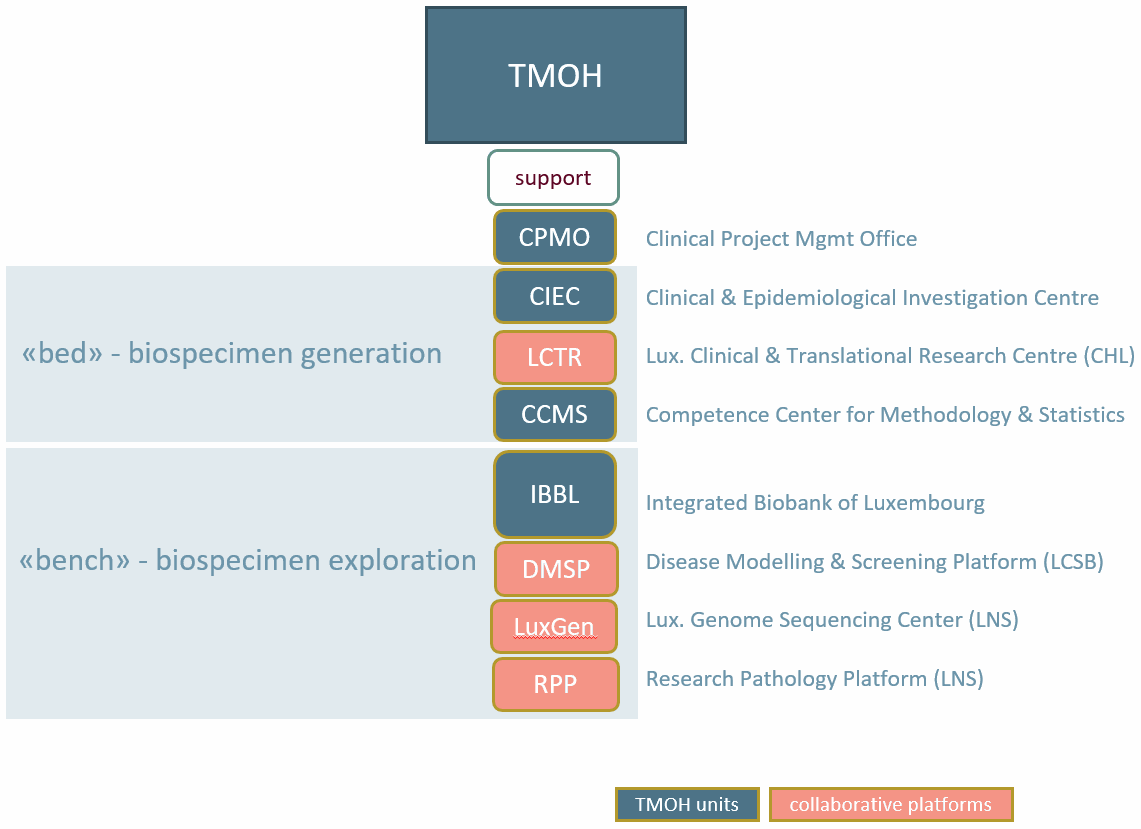
The central entry point for new projects is the CPMO, which will guide researchers and their projects through the governance and approval process, as well as plan and track project execution.
The TMOH facilitates intra-institutional research, as well as transversal translational projects of “Research Luxembourg” and collaborations with external partners.
These include publicly funded Research Projects like LITMUS, NCER-PD and CLINNOVA, as well as collaborations with industry partners.
Missions
TMOH - Research Services Dedicated to Life
The mission of TMOH is to improve patients’ lives by providing Operational Excellence:
Single Point of contact regarding Research Services for internal & external stakeholders
Seamless workflow and high-quality project execution
Consultation for projects regarding project design, operations, regulatory aspects, among others
Inbuilt collaboration (clear processes, interfaces, R&R) and exchange of resources (technologies, SME)
Transparent information/communication E2E (TMOH, LIH and beyond...)
Continuous development – of methods, technology, science, quality, and processes, among others
Head of department
The Department was established in July 2021, and Dr Hermann Thien was appointed as its Director. Hermann gained over 20 years of experience in the Pharmaceutical and Diagnostics Industry before joining LIH in March 2021.

