CANBIO - PhD Training Program
Individual projects - Induced Escape Mechanisms
The aim of the second work package is to propose and test novel strategies based on the identification of key resistance mechanisms of selected cancer types evolved in response to targeted therapies, DNA damaging agents or changing environments (e.g. hypoxia). These aspects will be studied using complementary molecular and cellular approaches and relevant pre-clinical animal models.
Understanding the underlying mechanisms that govern therapy-induced escape mechanisms of tumour cells is crucial to prevent tumour relapse and promote the rational design of combinatorial as well as personalised treatment approach.
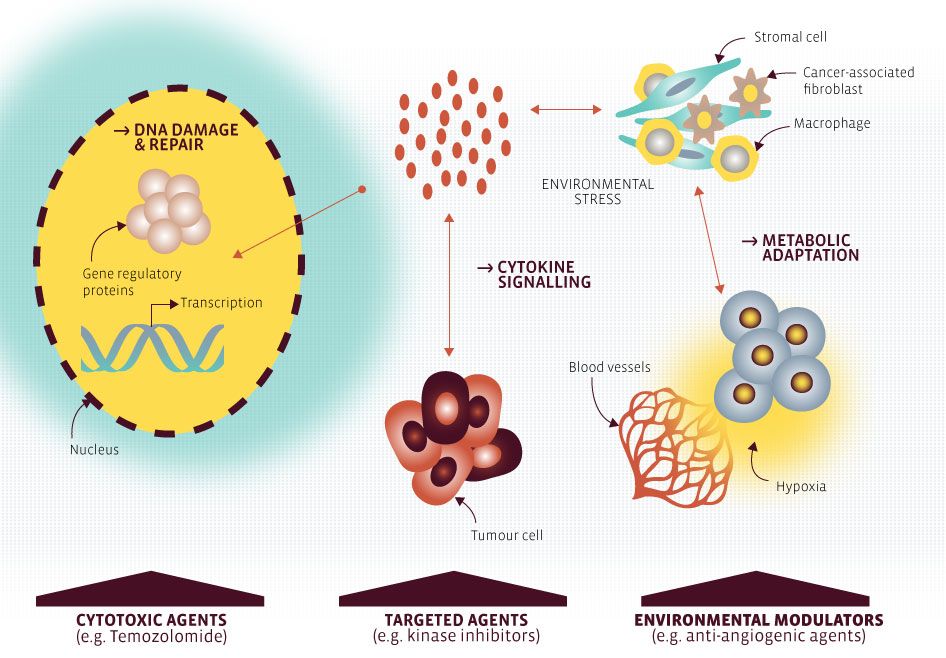
Project 1: Targeting DNA repair mechanisms in primary glioblastoma and their recurrences: from basic mechanisms to pre-clinical aspects and personalized therapy
- Supervision: Dr Eric van Dyck (LIH), Prof Iris Behrmann (UL)
Previous work has identified a list of DNA repair genes whose depletion in glioblastoma (GBM) cells leads to sensitivity to the DNA damaging agent temozolomide, and revealed gene-expression alterations that shape the “DNA repair makeup” of GBMs in clinical samples. This project will integrate in vitro approaches and the characterization of animal models of GBM to validate a selection of the most promising DNA repair factors in our list and test novel chemotherapeutic strategies against GBM.
Project 2: Targeting the Jak/STAT pathway in cancer: inhibitors and resistance mechanisms
- Supervision: Prof Iris Behrmann (UL)
The Jak/STAT pathway is often found constitutively active in cancer cells, either due to driver mutations affecting the signalling players or due to upregulation of activators, such as cytokines of the IL-6 family. This pathway can support a stemness phenotype of cancer cells and be involved in the development of chemoresistance, EMT and metastasis.
This project aims at investigating the molecular mechanisms underlying (cytokine)/Jak/STAT-mediated effects. Moreover, drugs targeting this pathway should be evaluated, including compounds already used in the clinic or in pre-clinical studies as well as novel drug candidates.
Project 3: Identifying and targeting the metabolic escape mechanisms in IDH mutant gliomas
- Supervision: Prof Simone Niclou (LIH)
The vast majority of low grade gliomas carries a mutation in the enzyme isocitrate dehydrogenase (IDH) which leads to the generation of the oncometabolite 2-hydroxyglutarate. We have used in situ metabolic profiling to determine the key metabolic aberrations in IDH mutant gliomas. Here we will determine the metabolic response and the escape mechanisms developed in gliomas after treatment with novel mutant IDH-inhibiting compounds.
Project 4: Endogenous molecular mechanisms of receptor tyrosine kinase inhibition and resistance in Glioblastoma
- Supervision: Prof Simone Niclou (LIH)
Our research focuses on the biology of malignant gliomas with the aim to elucidate novel treatment strategies. In this project, we will address the molecular mechanisms of an endogenous inhibitor of receptor tyrosine kinases (RTK) in Glioblastoma. LRIG1 protein is a pan-RTK regulator that potently inhibits GBM growth in vitro and in vivo. Here we aim to identify the active residue of LRIG1, its post-translational modifications and protein-protein interactions. This project will drive the design of a pan-RTK targeting approach in the context of GBM and resistance to anti-RTK therapy.
Project 5: Effect of environmental stress factors on colon cancer and its microenvironment
- Supervision: Prof Serge Haan (UL), Dr Bassam Janji (LIH/UPS)
Environmental stress conditions such as hypoxia influence tumour initiating cells and tumour-associated stromal cells. The project focusses on the role of hypoxia in tumor progression and the cross-talk between stromal cells and tumor-initiating cells. The study will take advantage of an in-house collection of patient-derived tumor sphere cultures and stromal cells and ultimately aims at the identification of factors that drive metastasis and chemoresistance.
Project 6: Signalling cross-talk between cytokines and environmental stress factors
- Supervision : Prof Iris Behrmann (UL)
The inflammatory cytokine Interleukin 6 (IL6) plays an important role in the development of hepatocellular carcinoma (HCC). It acts on the expansion of liver cancer stem cells, thereby also affecting metastasis formation and tumor recurrence after therapy. This project aims at identifying and targeting long non-coding RNAs involved in IL6/Jak/STAT3 signal transduction. The cross-talk between IL6 and hypoxic signaling pathways will also be addressed. Our study may help to develop therapeutic strategies targeting inflammatory networks in HCC.
DISCOVER CANBIO
About CANBIO - PhD Training Program
- WP1: Intrinsic Escape Mechanisms
- WP2: Induced Escape Mechanisms
- WP3: Disease Monitoring and Biological Networks
LuxDoc 3MT (Three Minute Thesis)
CONTACT
For any question related to CANBIO, please contact:
Prof Simone Niclou
canbio.office@lih.lu
Funded by

