6 November 2020
4 min read
[Press release] Advancing personalised cancer treatment through patient “avatars”
Luxembourg Institute of Health (LIH) establishes a collection of preclinical patient-derived brain tumour models to accelerate drug development
The NORLUX Neuro-Oncology Laboratory at the LIH Department of Oncology (DONC), in collaboration with the Centre Hospitalier de Luxembourg (CHL), the Laboratoire National de Santé (LNS), the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg and other international partners, established a large collection of patient-derived glioma organoids and xenograft models that mimic the specific features of patient brain tumours. These tools will allow the establishment of personalised drug efficacy studies, thus increasing the chances of success of clinical trials and improving clinical outcomes for patients. The findings were presented in a recent publication in the international peer-reviewed journal Acta Neuropathologica.
Clinical trials are crucial in order to test the efficacy of novel therapeutic molecules directly on patients. Nevertheless, their success is significantly dependent on the quality of the results obtained during preclinical drug testing studies carried out on experimental models of a patient’s tumour prior to the clinical phase. The availability of accurate and comprehensive models in the preclinical setting that reflect the full range of genetic variations observed in brain cancers and that are able to reliably predict the sensitivity of specific types of tumours to new personalised treatments is therefore of utmost importance in the context of translational oncology.
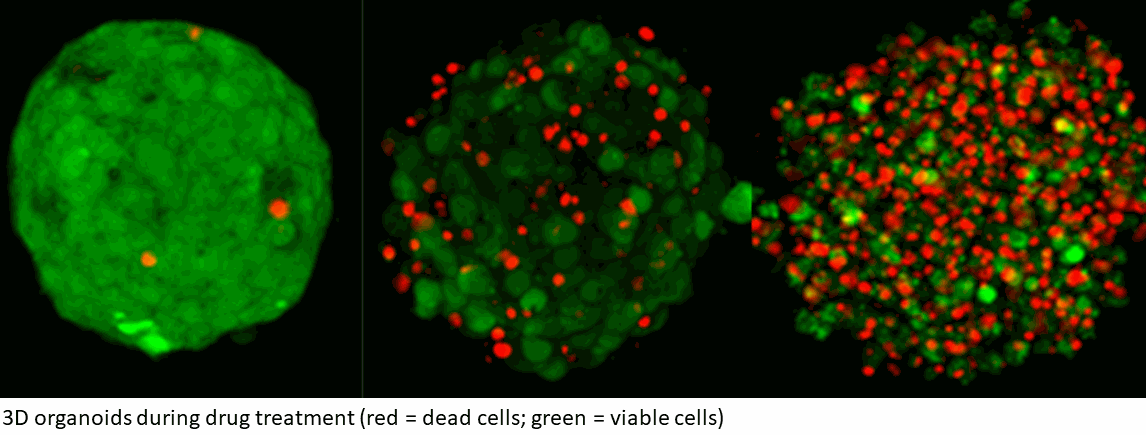
To address this currently unmet need, NORLUX has been working in close cooperation with the Centre Hospitalier de Luxembourg (CHL) and the major research institutes in Luxembourg to establish a collection of brain tumours from over 1000 patients. Through the samples provided by the Neurosurgery department of the CHL, NORLUX has been generating a biobank of brain tumour organoids – three-dimensional tissue cultures derived from viable cells from patient tumours. Tumor organoids are subsequently implanted in immunodeficient mice to give rise to so-called Patient-Derived Orthotopic Xenografts (PDOXs). NORLUX currently established a comprehensive cohort of over 40 of PDOXs from malignant gliomas including glioblastoma, one of the deadliest forms of brain cancer. These models act as clinically relevant patient ‘avatars’, faithfully reproducing the main biological, histological and genomic features of the original patient tumors. The cohort includes PDOXs from primary and recurrent gliomas, at different stages, from different subtypes and carrying different genetic mutations. Unique PDOXs derived from tumour samples of the same patient prior to and after treatment are also available, improving the understanding of how specific cancers respond to various treatments, according to their genetic characteristics.
“Brain cancer organoids and the resulting mouse avatars have a unique value as a drug screening platform in both preclinical and co-clinical studies. Concretely speaking, they can be leveraged in the context of precision oncology, such as in the ongoing clinical trial for Personalised Functional Profiling (PFP), led by LIH in collaboration with CHL, Hopitaux Robert Schuman (HRS), IBBL and LNS, as well as in research studies in partnership with pharmaceutical companies. Indeed, in our study we tested the efficacy of VAL-083, a DelMar Pharmaceuticals' compound, in our organoids and PDOXs, showing its potential as a promising candidate for glioblastoma”, states Dr Anna Golebiewska, Group Leader at NORLUX and co-first author of the scientific publication.
“Our work is the result of a distinctive interdisciplinary approach based on the complementary competences of Luxembourg’s biomedical research ecosystem”, explain Dr Ann-Christin Hau and Mrs Anaïs Oudin of the NORLUX research team and co-first authors of the publication. “Establishing such a biobank of preclinical models relied on the invaluable support of Prof F. Hertel and his team at the CHL Neurosurgery department, who were instrumental in enabling us to conduct the study. The LNS contributed through the renowned expertise of their National Centers of Pathology and of Genetics, LCSB performed state-of-the-art single cell transcriptomics, while the Clinical and Epidemiological Investigation Centre (CIEC) at LIH organised the collection of tissue samples while ensuring patient data confidentiality. Most importantly, we would like to extend our gratitude to all the patients and their families for making our study possible with their participation. We are convinced that our preclinical models will accelerate the translation of fundamental research findings into improved clinical treatments for future patients”, they add.
The collection of 40 PDOX glioma models and associated data is available to the international scientific community on PDXfinder, an open global catalogue co-developed by the European Molecular Biology Laboratory - European Bioinformatics Institute (EMBL - EBI) and the Jackson Laboratory.
“By sharing our models and data and joining forces with leading international partners in initiatives like the Horizon 2020-funded EurOPDX consortium and the Glioma Longitudinal AnalySiS (GLASS) consortium, we are continuously contributing to increasing the availability and use of these invaluable resources at the global level, thereby tangibly supporting breakthroughs in personalised cancer therapy”, concludes Prof Simone Niclou, Director of the LIH Department of Oncology (DONC) and corresponding author of the publication.
The findings were published in October 2020 in the international journal Acta Neuropathologica, with the full title “Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology”.