Predi-COVID - Behind the scenes
IBBL’s role in a COVID-19 cohort study
On April 24th, the Research Luxembourg Taskforce officially launched “Predi-COVID” (Luxembourg cohort of positive patients for COVID-19: a stratification study to predict severe prognosis), a study led by the Department of Population Health (DoPH) of the Luxembourg Institute of Health (LIH). Predi-COVID aims to set up a cohort of SARS-CoV-2 positive individuals with the goal of identifying important risk factors and biomarkers associated with COVID-19 severity and prognosis. The Integrated Biobank of Luxembourg (IBBL) has been playing a critical role in the project, leveraging on its established expertise in biological sample management and experienced team of motivated professionals.
Predi-COVID entails several key phases, which are crucial for the delivery of high-quality, reproducible and reliable results. Namely, these include patient recruitment, sample collection and logistics, sample processing and storage in a controlled environment. The complexity and diversity of these tasks require an array of different but complementary skills, which is where the collaborative effort of all project partners is the key. Indeed, each partner is responsible for a specific activity, contributing to the overall success of the operations. In this context, IBBL played a pivotal role in the design of the protocol and the sampling scheme, and is operationally responsible for the centralised management and transformation of biological samples, working in close cooperation with all other LIH departments involved in these stages and with the Luxembourg Centre for Systems Biomedicine (LCSB).
The first step of the project consists in recruiting COVID-19 patients and controls (i.e. members of the patient’s household) to build up the Predi-COVID cohort. This is performed by experienced nurses and clinical research associates from the Clinical and Epidemiological Investigation Center (CIEC) of the DoPH. If a subject is willing to participate in the study, the CIEC team contacts him/her to explain the study and organise an inclusion visit at home or at the hospital. During this visit, CIEC nurses verify the individual’s eligibility and collect his/her informed consent form, after which the sample and medical data collection process can begin. Since the beginning of the patient recruitment operations in early May, 36 donors have already been enrolled by the CIEC team.
For CIEC nurses to be able to obtain different types of biological samples from the study participants, specific kits need to be used, each of which containing various items needed for the different collection processes. IBBL’s Biorepository department (Biorep) — led by Katy Beaumont and comprising team members Laura Georges, Maeva Munsch, Margaux Henry, Florian Simon, Ana Festas and Nassima Ouzren — is responsible for the preparation of these collection kits and for their shipment to CIEC through a dedicated logistics company and according to well-defined standard procedures. In particular, for Predi-COVID, IBBL’s Biorep prepares blood collection kits consisting of five tubes with different reagents to stabilise different components of the blood; a tube to collect sputum samples; swabs with UTM tube to perform oropharyngeal and nasal specimen collections; and a stool collection kit. Owing to the meticulous and rigorous work of the Biorep team, a total of 190 kits have already been prepared and sent to CIEC since the start of project.
Once collected, samples are immediately transported back to CIEC by the nurses under particular temperature conditions. From there, they are further transferred to IBBL’s Biorep by Biologistic, a company specialised in the transport of health products, where they arrive on the same day at about 1pm. The Biorep team receives them, records them in the dedicated Laboratory Information Management System (LIMS) and deals with them according to their individual downstream uses, as follows:
Swab samples are sent to LIH House of Biohealth (HoBH) (Department of Infection and Immunity – DII) where they are processed by Chantal Snoeck and her team;
Blood tubes are sent to IBBL’s Biorefinery (Bioref) department (i.e. IBBL’s laboratory), for further processing;
Saliva (sputum) tubes are stored directly at -80°C in the Biorep;
Stool samples are sent by participants to Bioref by traditional mail
Upon arrival to IBBL Biorefinery, samples are processed, i.e. made amenable for further analyses and/or storage, by the dedicated Bioref team — Estelle Henry, Lucie Remark, Johanna Trouet, Brian De Witt, Geeta Acharya, Camille Bellora, Achilleas Pexaras, Pauline Lambert, Kate Sokolowska, Gael Hamot and Margaux Schmitt. Specifically, they undergo various procedures including separation – ensuring the differentiated recovery of different components of complex biological samples, aliquoting — which is the fractioning of each sample into smaller parts and different components — DNA and RNA extraction and quantification, as well as the manual isolation of peripheral blood mononuclear cells (PBMCs)[1], some of which are directly transferred to LIH for analysis. All samples are processed on the day of receipt to maximise their fitness-for-purpose for further analysis. This is made possible by the rapid, effective and accurate work of the entire Bioref team, under the coordination of Team Leaders Wim Ammerlaan and Alexander Hundt. To date, over 100 samples have been received and processed by the experienced team, resulting in over 1500 aliquots of serum, plasma, buffy coat, PBMCs, stool and PaxGene tubes, which are stored at IBBL. Almost all products are stored at -80°C for optimal preservation, with the exception of viable PBMCs, which are kept at -196°C in liquid nitrogen.
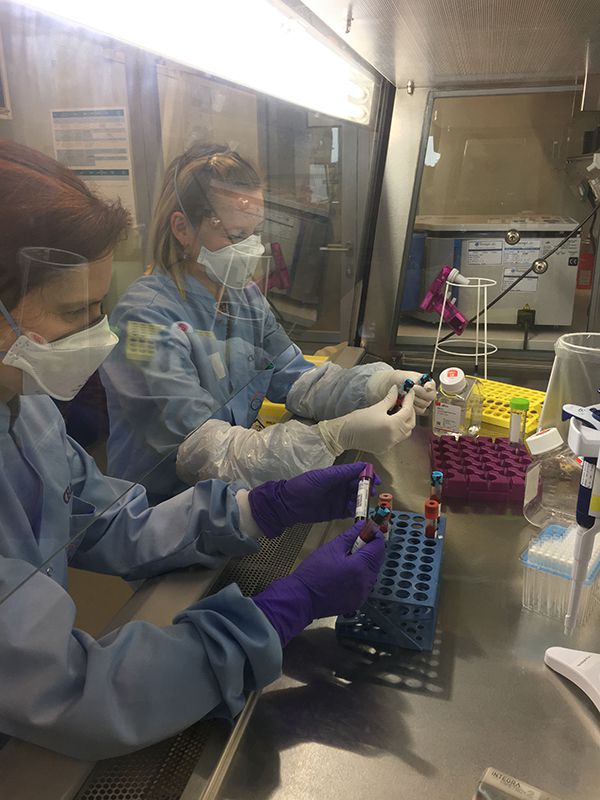
Blood sample processing at IBBL Bioref.

The Predi-COVID Bioref team (Achilleas Pexaras, Geeta Acharya, Pauline Lambert, Kate Sokolowska Wim Ammerlaan, Lucie Remark).
After being processed, the samples are ready to be distributed to the Predi-COVID partners for analysis, in order to investigate the ability of specific biomarkers from both the SARS-CoV-2 virus and the patient to predict disease severity. For instance, in terms of pathogen-related parameters, mutations of SARS-CoV-2 are analysed by the Laboratoire National de Santé (LNS) by sequencing the viral RNA isolated from nasal and oropharyngeal swabs, while the presence of other concomitant viral or bacterial infections is assessed by Chantal Snoeck and her team at the LIH DII by RT-PCR on nucleic acids extracted from swabs. The presence of SARS-CoV-2 and/or other viruses is also assessed in faeces samples by the Luxembourg Centre for Systems Biomedicine (LCSB) by analysing the extracted RNA. In terms of host-related markers, antibodies against SARS-CoV-2 in the serum derived from blood samples are detected by Chantal’s team to assess the patient’s immune response, while IBBL’s Bioref carries out HLA genotyping by sequencing the DNA extracted from blood samples to determine the patient’s HLA (human leukocyte antigen) genotype[2]. In addition, the IBBL team also performs complete blood count (CBC) to characterise the main immune cells present in the participant’s blood.
“IBBL acts as a highly precise and well-orchestrated machinery, making sure that the project’s most valuable assets – biological samples – are transported, processed, stored, distributed and analysed under monitored conditions, within short timeframes and according to strict standard operating procedures”, says Katy Beaumont, Head of Biorepository at IBBL. “This ensures they are fit for the purpose of downstream analyses, which in turn will allow us to generate meaningful findings”, add Wim Ammerlaan and Alexander Hundt. “The effective management of biological samples could not be possible without the motivation and commitment of the entire IBBL staff – from Biorep and Bioref, to project management, IT and administration – who is working relentlessly in the fight against COVID-19 in close collaboration with the DoPH and DII teams to provide concrete benefits for Luxembourg patients”, concludes Prof Laetitia Huiart, Director of the LIH Department of Population Health and Principal Investigator of the study.
1 Any blood cell having a round nucleus, such as lymphocytes, monocytes or macrophages.
2 A group of proteins expressed on the surface of human cells which influence the virus antigen presentation and specificity of the immune response.



