Master detox molecule boosts immune defence
LIH scientists have discovered a so far unknown molecular mechanism promoting the activation of the human immune system. The team, led by Prof Dirk Brenner, FNR ATTRACT fellow and Head of the Experimental & Molecular Immunology research group, has been studying the glutathione molecule, produced among others T cells and known for its role in cleaning the body from harmful metabolic wastes. Their research project revealed that glutathione also stimulates T cells energy metabolism.
To make it simple, glutathione is key in helping T cells getting enough energy to grow, divide and fight off intruders when in contact with pathogens such as viruses for example. It is thus essential for an optimal immune response. This discovery offers starting points and perspectives to develop new therapeutic strategies for targeting cancer and autoimmune diseases. The scientists publish their findings today in the world’s most prestigious immunology journal “Immunity".
"Our body has to keep our immune system in a carefully balanced equilibrium”, says Prof Dirk Brenner. “If the body’s innate defences are overactive, then they turn against the body. This is what happens in autoimmune diseases like multiple sclerosis or arthritis, for example. However if the defences are too weak, then infections cannot be handled or body cells can proliferate uncontrolled and grow to form tumours, which can become life threatening.” Immune cells such as T cells therefore normally reside in a state of alert hibernation, with their energy consumption reduced to a minimum. If pathogens get in contact with the T cells, these wake up and boost their metabolism, to produce more energy. This necessarily creates greater amounts of metabolic waste products such as reactive oxygen species (ROS) and free radicals, which can be toxic for the cells.
When the concentration of these oxidants increases, the T cells have to produce more antioxidants so as not to be poisoned. No previous research group had studied the mechanism of action of antioxidants in T cells to great detail before. In exploring this phenomenon, Prof Brenner’s team discovered that the antioxidant glutathione produced by T cells serves not only as a garbage collector to dispose of metabolic waste products, it is also a key switch for energy metabolism that controls the immune response, and is thus of high relevance to various diseases.
“These fascinating results form a basis for a targeted intervening in the metabolism of immune cells and for developing a new generation of immunotherapies,” explains Prof Markus Ollert, Director of LIH’s Department of Infection and Immunity.
For their investigations, the scientists employed animal models having T cells unable to produce glutathione. “In these mice, we discovered that the control of viruses is impaired – these mice have an immunodeficiency. But by the same token, this also meant the mice could not develop any autoimmune disease such as multiple sclerosis.” Further tests performed by Prof Brenner’s team demonstrated the reason for this: “The mice cannot produce any glutathione in their T cells”, Brenner continues, “As a result, without glutathione, T cells do not become fully functional; they remain in their state of hibernation and no self-destructive autoimmune response occurs.” Prof Karsten Hiller from the Braunschweig University of Technology who collaborated with the Luxembourgish scientists adds: “It is intriguing to see that cellular metabolism and immune activation are so tightly entangled and that a fine-grained interplay is essential to achieve a correct function."
Prof Dirk Brenner sees his T cell experiments as a prelude to more in-depth investigation of the energy balance of immune cells in general. A number of different autoimmune diseases, for example, are related to malfunctions in various subgroups of T cells. “If we understand the differences in the molecular mechanisms by which they stimulate their metabolism to get energy during defensive or autoimmune responses, then we can discover clues as to possible attack points for therapeutic agents regulating the immune response.” The distinguished researcher sees a similar situation in cancer: “In this context too, it is important to know why the immune cells that are actually supposed to fight cancer cells drop to a low metabolic state and in some cases even actively suppress an immune response against the tumour. Counteractive metabolism-stimulating measures could make the immune cells work more efficiently and fight off cancer more effectively.”
Link to publication: Glutathione Primes T Cell Metabolism for Inflammation
Link to article featuring publication: Caught in the cROSsfire: GSH Controls T Cell Metabolic Reprogramming

Prof Dirk Brenner (principal investigator and corresponding author on the Immunity publication) and Dr Melanie Grusdat (first author) in the laboratory.
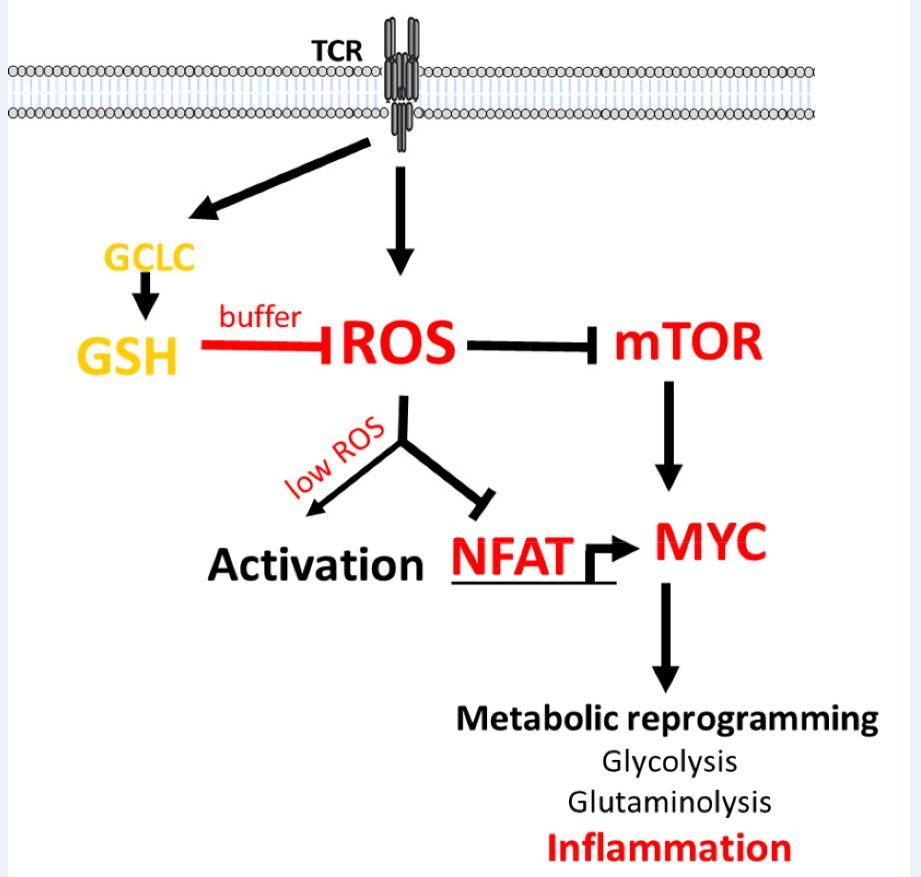
Mechanism of action of glutathione on metabolic reprogramming
