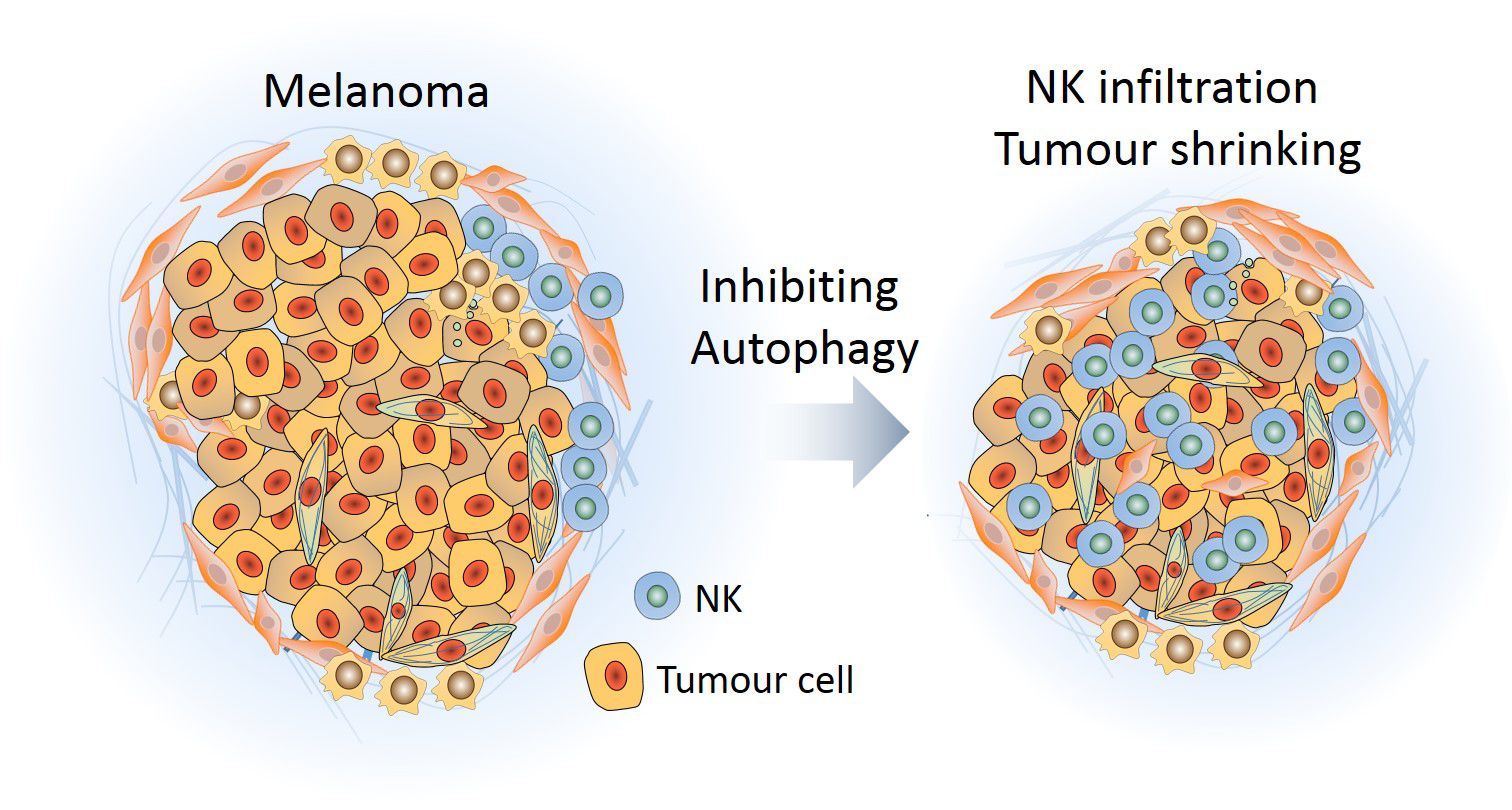
Natural Killer (NK) cells, lymphocytes of the innate immune system with strong cytotoxic activity, play a major role in the immune response against tumours. However, tumour cells can circumvent this immune defence by establishing a surrounding microenvironment that prevents the infiltration of NK cells and thus promotes tumour survival and growth. By studying melanoma, a highly malignant form of skin cancer, Dr Bassam Janji's research team at LIH has revealed a mechanism by which the immunosuppressive environment can be switched to an immunosupportive one. The researchers found that if autophagy - the intracellular recycling process - is blocked in tumour cells, they produce cytokines that attract NK cells. The massive recruitment of NK cells allows killing cancer cells and lets the tumours shrink.
The scientists published their findings in open access in the October issue of the acclaimed scientific journal “Proceedings of the National Academy of Sciences of the United States of America” (PNAS).
A critical hallmark of the malignant progression of tumours is evasion and suppression of the immune system. This occurs through the ability of tumour cells to develop an immunosuppressive microenvironment preventing cytotoxic immune cells such as NK cells to infiltrate tumours and kill cancer cells. Therefore, a key issue in the field of anti-cancer immunotherapy is to develop strategies capable of driving cytotoxic immune cells into the tumour bed.
For several years already, it is well known that tumour growth can be reduced by suppressing autophagy in cancer cells. While autophagy blockade is believed to render tumours more sensitive to chemotherapy, its impact on anti-tumour immunity is not well understood. Autophagy can be blocked in tumour cells and mouse models pharmacologically and genetically, the latter by inhibiting the expression of genes involved in the initiation of autophagy, for example BECN1 coding for the protein Beclin-1.
Targeting autophagy impacts on NK cells
The research team of Dr Bassam Janji in the Laboratory of Experimental Cancer Research at LIH’s Department of Oncology is specialised in the study of the tumour microenvironment in different types of solid tumours, including skin cancer. In their newly published study, the researchers aimed to investigate the effect of blocking autophagy on the infiltration of NK cells in melanoma.
They found that, when the autophagy process is blocked in tumour cells by inhibiting the expression of BECN1, a large amount of functional NK cells infiltrated into the tumour. This led to a significant reduction in tumour size. The research team revealed that autophagy-defective tumour cells produced an increased amount of CCL5, a small cytokine with chemotactic properties, able to attract NK cells to the tumour bed. When CCL5 is depleted, the infiltration of NK cells and the subsequent regression of tumour volume were no longer observed, thus confirming the crucial role of CCL5 in driving NK cells into autophagy-defective tumours. The researchers further elucidated that the increased production of CCL5 in the absence of autophagy is due to the activation of the kinase JNK and the transcription factor c-Jun.
Using tumour biopsies from melanoma patients, the researchers could show that there is a positive correlation between the production of CCL5 and the infiltration of NK cells. The more CCL5 is produced, the stronger tumours are infiltrated with NK cells. A high level of production of CCL5 was also found to have a positive impact on the survival of melanoma patients.
Directing NK cells to the tumour
This is the first time that a mechanistic link between autophagy and NK cell recruitment could be established. Dr Janji sees a high therapeutic potential in the discovery. 'Targeting autophagy in tumour cells is a promising strategy to reinforce the immune system to fight cancer', he claims. 'Our study provides a cutting edge advance in the field of cancer immunotherapy and could specifically pave the way for more effective NK cell-based immunotherapies. We now know how we can switch an immunosuppressive tumour environment to an immunosupportive one, enabling the action of NK cells. NK cells have a strong anti-tumour potential, but it can only be exploited if the cells are efficiently directed to the tumour.'
The researchers are now planning to address the impact of targeting autophagy either genetically or pharmacologically on the immune landscape of melanomas by phenotyping other immune cells in the melanoma tumour microenvironment. The ultimate goal of Dr Janji’s research team is to provide the proof of concept that targeting autophagy could improve the efficacy of current immunotherapies, notably those based on immune checkpoint blockades.
Involved research teams and funding
The research project was carried out by members of the Laboratory of Experimental Cancer Research at LIH’s Department of Oncology under the lead of Dr Janji, Deputy Head of Unit. The research unit worked in close collaboration with other research entities in Luxembourg: the National Cytometry Platform within LIH’s Department of Infection and Immunity, the Life Sciences Research Unit of the University of Luxembourg, the National Health Laboratory “Laboratoire national de santé” and the major local hospital “Centre Hospitalier de Luxembourg”. Valuable partners in the project were as well the renowned Gustave Roussy Cancer Centre (INSERM UMR 981 and 1186) in Villejuif, France, and the University Hospital of Besançon, France.
The project was generously supported by grants from the Luxembourg National Research Fund (FNR), F.R.S.-FNRS Télévie, the Luxembourg Cancer Foundation “Fondation Cancer”, the Calouste Gulbenkian Foundation, “Ligue contre le Cancer”, the French National Cancer Institute and intramural funding.
Mgrditchian et al., 2017 (doi: 10.1073/pnas.1703921114)
Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner.
http://www.pnas.org/content/114/44/E9271.long
A press release is available in French and German.